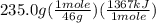Chemistry, 13.07.2019 01:00 marvinc5603
The combustion of one mole of liquid ethanol, ch3ch2oh, produces 1367 kj of heat. calculate how much heat is produced when 235.0 g of ethanol are combusted.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
The combustion of one mole of liquid ethanol, ch3ch2oh, produces 1367 kj of heat. calculate how much...
Questions in other subjects:







Physics, 04.09.2020 18:01

Mathematics, 04.09.2020 18:01

Mathematics, 04.09.2020 18:01

Health, 04.09.2020 18:01