
Chemistry, 21.09.2019 18:20 alondrachon
Citric acid is composed of only carbon, hydrogen, and oxygen. when a 0.5000 g sample of citric acid was burned, it produced 0.6871 g of co2 and 0.1874 g of h2o. the molar mass of the compound is 192 g/mol. what are the empirical and molecular formulas of citric acid

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Citric acid is composed of only carbon, hydrogen, and oxygen. when a 0.5000 g sample of citric acid...
Questions in other subjects:

Mathematics, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01



Social Studies, 20.08.2020 01:01



Business, 20.08.2020 01:01


Mathematics, 20.08.2020 01:01

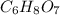 is empirical formula as well as molecular formula.
is empirical formula as well as molecular formula.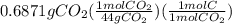
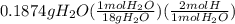
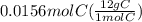
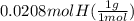
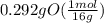
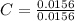 = 1
= 1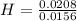 = 1.33
= 1.33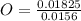 = 1.17
= 1.17

