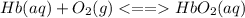Chemistry, 13.07.2019 06:30 darkremnant14
What happens to the ratio of oxygenated hbo2(aq) to deoxygenated hb(aq), i. e. , when there is a high pressure of oxygen, po2(g), in the lungs?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20heldmadison
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
What happens to the ratio of oxygenated hbo2(aq) to deoxygenated hb(aq), i. e. , when there is a hig...
Questions in other subjects:



Mathematics, 23.05.2021 01:50


Business, 23.05.2021 01:50

Mathematics, 23.05.2021 01:50

English, 23.05.2021 01:50



Physics, 23.05.2021 01:50