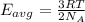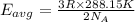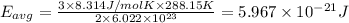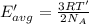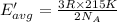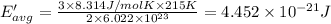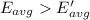How does a sample of helium at 15 °c compare to a sample of helium at 215 k? the helium at 15 °c has a higher average kinetic energy than the sample at 215 k. the helium at 15 °c has lower nuclear energy than the sample at 215 k. the helium at 15 °c has slower-moving atoms than the sample at 215 k. the helium at 15 °c has smaller atoms than the sample at 215 k.

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
How does a sample of helium at 15 °c compare to a sample of helium at 215 k? the helium at 15 °c ha...
Questions in other subjects:







Computers and Technology, 25.11.2021 05:10