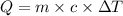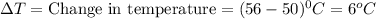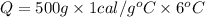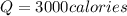Chemistry, 13.07.2019 11:30 lisapcarroll
How many calories are absorbed by a sample of water with a mass of 500 grams as it changes from 50°c to 56°c? the specific heat capacity of water is 1.00 cal/g°c. 83 calories 494 calories 506 calories 3,000 calories

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
How many calories are absorbed by a sample of water with a mass of 500 grams as it changes from 50°c...
Questions in other subjects:



Mathematics, 27.10.2020 23:00


English, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00

Biology, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00


Mathematics, 27.10.2020 23:00