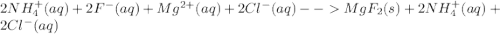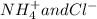Write the net ionic equation for this reaction occurring in water: ammonium fluoride and magnesium chloride are mixed to form magnesium fluoride and ammonium chloride. 1. no reaction occurs. 2. 2 nh+ 4 + 2 cl− → 2 nh4cl 3. 2 f− + mg2+ → mgf2 4. 2 nh4f + 2 cl− → 2 f− + 2 nh4cl 5. 2 f− + mgcl2 → mgf2 + 2 cl−

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3
You know the right answer?
Write the net ionic equation for this reaction occurring in water: ammonium fluoride and magnesium...
Questions in other subjects:

English, 14.04.2021 04:30


Mathematics, 14.04.2021 04:30

History, 14.04.2021 04:30


English, 14.04.2021 04:30

Social Studies, 14.04.2021 04:30


Mathematics, 14.04.2021 04:30

Mathematics, 14.04.2021 04:30

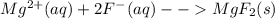

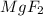 will not split into ions as it is insoluble in water.
will not split into ions as it is insoluble in water.