Chemistry, 13.07.2019 11:30 heggestade
A100 gram glass container contains 200 grams of water and 50.0 grams of ice all at 0°c. a 200 gram piece of lead at 100°c is added to the water and ice in the container. what is the final temperature of the system? (specific heat of ice = 2,000 j/kg°c , specific heat of water = 4,186 j/kg°c, heat of fusion of water = 333.7 kj/kg, specific heat of glass = 837.2 j/km°c, specific heat of lead = 127.7 j/km°c)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
A100 gram glass container contains 200 grams of water and 50.0 grams of ice all at 0°c. a 200 gram p...
Questions in other subjects:

Chemistry, 27.07.2019 02:30

English, 27.07.2019 02:30






Chemistry, 27.07.2019 02:30


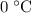
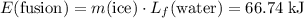 and
and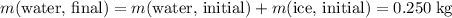
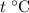 . Thus
. Thus 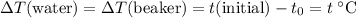
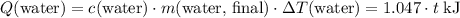 (converted to kilojoules)
(converted to kilojoules) 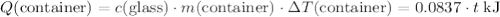
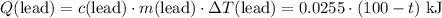
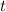 .
. 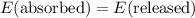
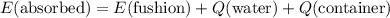
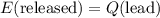
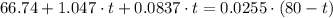
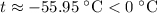 which goes against the initial assumption. Implying that the final temperature does not go above the melting point of water- i.e.,
which goes against the initial assumption. Implying that the final temperature does not go above the melting point of water- i.e., 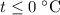 . However, there's no way for the temperature of the system to go below
. However, there's no way for the temperature of the system to go below 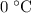 ; doing so would require the removal of heat from the system which isn't possible under the given circumstance; the ice-water mixture experiences an addition of heat as the hot block of lead was added to the system.
; doing so would require the removal of heat from the system which isn't possible under the given circumstance; the ice-water mixture experiences an addition of heat as the hot block of lead was added to the system.