

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 23.06.2019 06:50, summerjoiner
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1

You know the right answer?
An atom emits of wavelength 1.08 meters. what is the energy change occurring in the atom due to this...
Questions in other subjects:

Health, 27.04.2021 01:00




Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Spanish, 27.04.2021 01:00



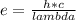
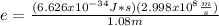
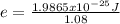
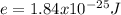
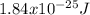 .
.

