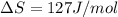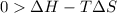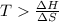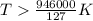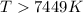Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1


Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 17:30, dalejacksoniip5yf4y
Hydrogen-2 is also known as deuterium as well as hydrogen-3 is known as tritium hydrogen-1 is our common hydrogen isotope a sample hydrogen gas has 99% hydrogen -1 ,0.8% deuterium , and 0.2% tritium what is the average atomic mass of this mixture of isotope to the thousands place
Answers: 1
You know the right answer?
At what temperature is the following reaction feasible: hcl(g) + nh3(g) -> nh4cl(s)?
Questions in other subjects:

Mathematics, 02.11.2020 23:00



Mathematics, 02.11.2020 23:00


Social Studies, 02.11.2020 23:00


Mathematics, 02.11.2020 23:00


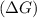 should be negative or
should be negative or 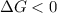 .
.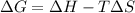
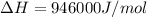 and
and