Chemistry, 13.07.2019 16:30 rerunkle96
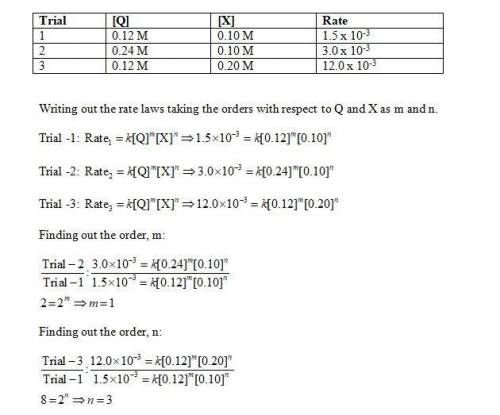
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. q + x yields products trial [q] [x] rate 1 0.12 m 0.10 m 1.5 × 10-3 m/min 2 0.24 m 0.10 m 3.0 × 10-3 m/min 3 0.12 m 0.20 m 12.0 × 10-3 m/min

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions in other subjects:


Mathematics, 26.10.2021 14:00



Mathematics, 26.10.2021 14:00

Mathematics, 26.10.2021 14:00