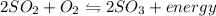Chemistry, 13.07.2019 18:00 Woodsydal2390
Which situation would cause the following equilibrium reaction to decrease the formation of the products? 2so2 (g) + o2 (g) two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2so3 (g) + energy increase the temperature decrease the volume increase the pressure decrease the temperature

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1


Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Which situation would cause the following equilibrium reaction to decrease the formation of the prod...
Questions in other subjects:

English, 02.04.2021 17:40

Geography, 02.04.2021 17:40

Health, 02.04.2021 17:40

Mathematics, 02.04.2021 17:40

Mathematics, 02.04.2021 17:40

Biology, 02.04.2021 17:40

Mathematics, 02.04.2021 17:40



English, 02.04.2021 17:40