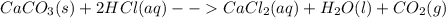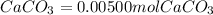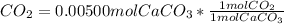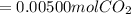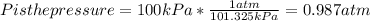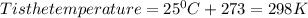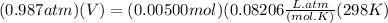Chemistry, 13.07.2019 18:30 auzriannamarie
Chris the chemist works in a laboratory in which the temperature is maintained at a constant 25oc and the pressure is always 100 kpa. chris needs to analyse some calcium carbonate, caco3(s), to determine whether it is pure or has been contaminated. chris will analyse the calcium carbonate by taking a small 0.00500 mole sample and adding hydrochloric acid, hcl(aq), to it until all the calcium carbonate has disappeared and no more carbon dioxide gas, co2(g), is produced. as the gas is produced it will be collected by a water displacement method. the balanced chemical equation for this reaction is known to be: caco3(s) + 2hcl(aq) → cacl2(aq) + co2(g) + h2o(l) if the sample is pure, what volume of carbon dioxide gas will be collected?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2
You know the right answer?
Chris the chemist works in a laboratory in which the temperature is maintained at a constant 25oc an...
Questions in other subjects: