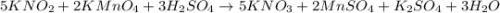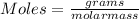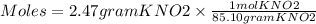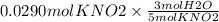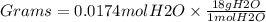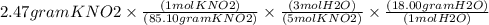Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3


Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Using the following equation 5kno2+2kmno4+3h2so4=5kno3+2mnso4+k2 so4+3h20. starting with 2.47 grams...
Questions in other subjects:






Mathematics, 15.08.2021 07:10

Mathematics, 15.08.2021 07:10

History, 15.08.2021 07:10


Chemistry, 15.08.2021 07:10