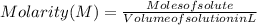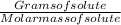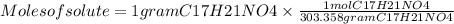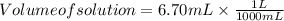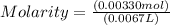Chemistry, 13.07.2019 20:00 myrkaxsanchezz
The free-base form of cocaine has a solubility of 1.00 g in 6.70 ml ethanol (ch3ch2oh). calculate the molarity of a saturated solution of the free-base form of cocaine in ethanol.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
The free-base form of cocaine has a solubility of 1.00 g in 6.70 ml ethanol (ch3ch2oh). calculate th...
Questions in other subjects:


Mathematics, 19.02.2021 23:30



Mathematics, 19.02.2021 23:30

Social Studies, 19.02.2021 23:30



Mathematics, 19.02.2021 23:30

Mathematics, 19.02.2021 23:30