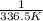Chemistry, 13.07.2019 21:30 thomasg185
The vapor pressure of ethanol is 400. mmhg at 63.5°c. its molar heat of vaporization is 39.3 kj/mol. what is the vapor pressure of ethanol, in mmhg, at 34.9°c? (r = 8.314 j/k • mol)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

You know the right answer?
The vapor pressure of ethanol is 400. mmhg at 63.5°c. its molar heat of vaporization is 39.3 kj/mol....
Questions in other subjects:

History, 14.11.2019 19:31

Mathematics, 14.11.2019 19:31


Mathematics, 14.11.2019 19:31

Arts, 14.11.2019 19:31

Mathematics, 14.11.2019 19:31

History, 14.11.2019 19:31



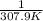 -
-