
Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: what is the enthalpy change when 2.50 mol of nitrogen dioxide decomposes? 13.5 kj of energy released 13.5 kj of energy absorbed 84.6 kj of energy released 84.6 kj of energy absorbed

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 06:00, mirzakasumovic8926
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: wh...
Questions in other subjects:

English, 03.03.2020 00:04










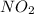 is formed = 33.84 kJ
is formed = 33.84 kJ


