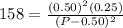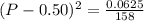Chemistry, 13.07.2019 22:00 elijahdouglass00
Asample of pure no2 gas decomposes at 1000 k 2no2 (g) ↔ 2 no (g) + o2 (g) the constant kp is 158. an analysis shows that the partial pressure of o2 is 0.25 atmospheres at equilibrium. determine the pressure of no and no2.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1
You know the right answer?
Asample of pure no2 gas decomposes at 1000 k ...
Questions in other subjects:

Mathematics, 14.06.2020 15:57

Social Studies, 14.06.2020 15:57


Computers and Technology, 14.06.2020 15:57

Mathematics, 14.06.2020 15:57

Mathematics, 14.06.2020 15:57

Computers and Technology, 14.06.2020 15:57


Physics, 14.06.2020 15:57

 = 0.02 atm.
= 0.02 atm.
![Kp=(\frac{[NO]^2[O_2]}{[NO_2]^2})](/tpl/images/0086/2744/569f6.png)