
Chemistry, 14.07.2019 02:00 autumperry7078
The energy from 0.02 moles of butane is used to heat 328 grams of water. the temperature of the water rose from 298 k to 343 k. (the specific heat capacity of water is 4.18 j/k g.) what is the enthalpy of combustion? a. -61.7 kj b. 1,578.01 j c. 3,084,840.0 j d. 23,513,336 j

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The energy from 0.02 moles of butane is used to heat 328 grams of water. the temperature of the wate...
Questions in other subjects:

Chemistry, 05.03.2021 19:50

Mathematics, 05.03.2021 19:50




Computers and Technology, 05.03.2021 19:50




Chemistry, 05.03.2021 19:50

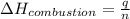
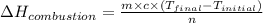
 = enthalpy of combustion = ?
= enthalpy of combustion = ? = specific heat of water=
= specific heat of water= 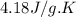
 = final temperature = 343 K
= final temperature = 343 K = initial temperature = 298 K
= initial temperature = 298 K



