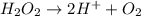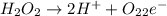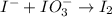Chemistry, 14.07.2019 02:30 madisonrparks
Question 8 how many electrons should be added to balance the following oxidation half-reaction, h2o2 imported asset 2 h+ + o2? none one two four question 9 what are the appropriate balanced half-reactions for the following reaction, which is carried out in an acidic solution: i− + io3− imported asset i2 ? i− imported asset i2 (oxidized) and io3 imported asset i2 (reduced) io3 imported asset i2 (oxidized) and i− imported asset i2 (reduced) 2 i− imported asset i2 + 2 e− (oxidized) and 2 io3− + 2 e− imported asset i2 (reduced) 2 i− imported asset i2 + 2 e− (oxidized) and 2 io3− + 10 e− imported asset i2 (reduced)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2



Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
Question 8 how many electrons should be added to balance the following oxidation half-reaction, h2o2...
Questions in other subjects:

Mathematics, 20.08.2019 00:00

Mathematics, 20.08.2019 00:00

Mathematics, 20.08.2019 00:00

Physics, 20.08.2019 00:00

Mathematics, 20.08.2019 00:00

Geography, 20.08.2019 00:00



Geography, 20.08.2019 00:00

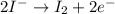 (oxidized) and
(oxidized) and 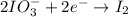 (reduced)
(reduced)