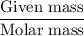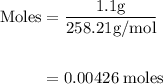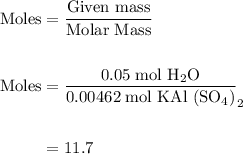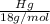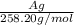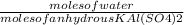Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
B) calculate the ratio of moles of h2o to moles of anhydrous kal(so4)2. note: report the ratio to t...
Questions in other subjects:

Business, 04.12.2020 02:00

Mathematics, 04.12.2020 02:00


History, 04.12.2020 02:00



Mathematics, 04.12.2020 02:00


English, 04.12.2020 02:00

Biology, 04.12.2020 02:00

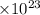 particles. In the given question, ratio of moles of H
particles. In the given question, ratio of moles of H O to the moles of anhydrous KAl(SO
O to the moles of anhydrous KAl(SO )
)