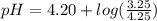Do parts a, b and c a. calculate the ph of 100 ml of an aqueous solution of 0.05 m benzoic acid and 0.025 m benzoate ion. the ka of benzoic acid is 6.3 x 10-5 at 25 ℃. b. calculate the new ph of the solution in part a if you add 10.0 ml of 0.100 m hcl solution. c. calculate the new ph of the solution in part a if you add 15.0 ml of 0.050 m naoh solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
Do parts a, b and c a. calculate the ph of 100 ml of an aqueous solution of 0.05 m benzoic acid and...
Questions in other subjects:





Mathematics, 22.08.2020 23:01



Biology, 22.08.2020 23:01



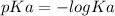
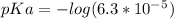
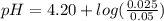
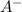 .
.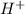 added = 0.100(10.0) = 1
added = 0.100(10.0) = 1

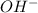 added to the original buffer = 0.05(15.0) = 0.75
added to the original buffer = 0.05(15.0) = 0.75