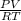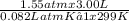Chemistry, 14.07.2019 04:30 briannaleiigh
A3.00-l flask is filled with gaseous ammonia, nh3. the gas pressure measured at 26.0 ∘c is 1.55 atm . assuming ideal gas behavior, how many grams of ammonia are in the flask?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 14:50, montanolumpuy
Select the correct answer from each drop-down menu. in the process of nuclear fission, . fission only happens to very atoms. the fission process usually also produces several free .
Answers: 2
You know the right answer?
A3.00-l flask is filled with gaseous ammonia, nh3. the gas pressure measured at 26.0 ∘c is 1.55 atm...
Questions in other subjects:


English, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31



Biology, 11.11.2019 22:31


Mathematics, 11.11.2019 22:31

Mathematics, 11.11.2019 22:31