
Chemistry, 14.07.2019 06:00 achoward08
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reaction is at dynamic equilibrium, at what rate will substance b be consumed? 0.0 mol/l·s 1.0 mol/l·s 2.0 mol/l·s 4.0 mol/l·s

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2


Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
In the hypothetical reaction below, substance a is consumed at a rate of 2.0 mol/l·s. if this reacti...
Questions in other subjects:

Mathematics, 02.07.2019 19:40


Mathematics, 02.07.2019 19:40

Biology, 02.07.2019 19:40

Mathematics, 02.07.2019 19:40

Mathematics, 02.07.2019 19:40

Chemistry, 02.07.2019 19:40

Mathematics, 02.07.2019 19:40


Chemistry, 02.07.2019 19:40

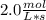
![R(a) = k * \frac{d[A]}{dt}](/tpl/images/0087/5945/2a16d.png)
![R (b)= k * \frac{d[B]}{dt}](/tpl/images/0087/5945/bbb74.png)


