
Chemistry, 14.07.2019 06:00 miathegreat
In a chemical reaction that takes place at a fixed pressure and volume, the enthalpy change (δh) is –585 kj/mol. will this reaction result in an increase or a decrease in temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
In a chemical reaction that takes place at a fixed pressure and volume, the enthalpy change (δh) is...
Questions in other subjects:

Chemistry, 30.11.2021 05:40


Mathematics, 30.11.2021 05:40

Spanish, 30.11.2021 05:40


Mathematics, 30.11.2021 05:40

World Languages, 30.11.2021 05:40

History, 30.11.2021 05:40


Mathematics, 30.11.2021 05:40

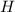 is negative it means an exothermic reaction where the heat is lose so the temperature decreases.
is negative it means an exothermic reaction where the heat is lose so the temperature decreases.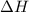 for the reaction comes out to be positive. The energy is absorbed and hence the temperature of the surroundings decrease.
for the reaction comes out to be positive. The energy is absorbed and hence the temperature of the surroundings decrease.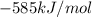 , the tempearture increase.
, the tempearture increase.

