
Chemistry, 14.07.2019 08:00 varonadestiny1109
How many moles of chlorine (cl) atoms are in a sample of 1.72 × 1022 atoms? 0.0286 mol cl 35.0 mol cl 1.03 × 1023 mol cl 1.04 × 1046 mol cl

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
How many moles of chlorine (cl) atoms are in a sample of 1.72 × 1022 atoms? 0.0286 mol cl 35.0 mol...
Questions in other subjects:

English, 11.02.2021 06:10


Mathematics, 11.02.2021 06:10

History, 11.02.2021 06:10

Mathematics, 11.02.2021 06:10

Social Studies, 11.02.2021 06:10

Geography, 11.02.2021 06:10

Mathematics, 11.02.2021 06:10

Mathematics, 11.02.2021 06:10

Mathematics, 11.02.2021 06:10

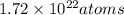
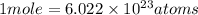
 is Avogadro number.
is Avogadro number.



