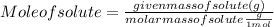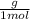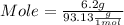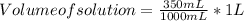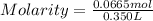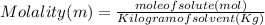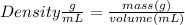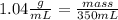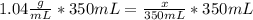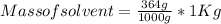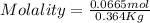Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Astudent dissolves 6.2g of aniline c6h5nh2 in 350.ml of a solvent with a density of 1.04/gml . the s...
Questions in other subjects:

English, 26.04.2020 01:55

Physics, 26.04.2020 01:55

Mathematics, 26.04.2020 01:55

Mathematics, 26.04.2020 01:55

History, 26.04.2020 01:55

Social Studies, 26.04.2020 01:55




Mathematics, 26.04.2020 01:55

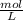 and Molality = 0.18
and Molality = 0.18 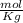 .
.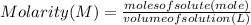 \
\