
Chemistry, 14.07.2019 09:00 baltazmapa629n
Isopropyl alcohol is mixed with water to produce a 37.0% (v/v) alcohol solution. how many milliliters of each component are present in 835 ml of this solution? (assume that volumes are additive.)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Isopropyl alcohol is mixed with water to produce a 37.0% (v/v) alcohol solution. how many milliliter...
Questions in other subjects:

Mathematics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01



Mathematics, 12.10.2020 01:01

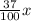 835 mL
835 mL

