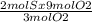Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3


Chemistry, 23.06.2019 08:00, mantha0402
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1

Chemistry, 23.06.2019 10:30, mikemofun9079
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
Consider the reaction between reactants s and o2: 2s(s)+3o2(g)→2so3(g)if a reaction vessel initiall...
Questions in other subjects:

Mathematics, 13.04.2021 19:30

Mathematics, 13.04.2021 19:30


Business, 13.04.2021 19:30

Chemistry, 13.04.2021 19:30





Mathematics, 13.04.2021 19:30