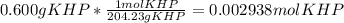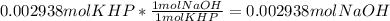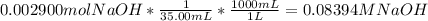Chemistry, 14.07.2019 12:00 potatogirl6811
Reagent grade potassium hydrogen phthalate (khp, mass 204.23g/mole) is a high molecular weight, stable, monoprotic solid acid. it is commonly used for standardizing sodium hydroxide solutions. what concentration of sodium hydroxide solution would be needed to titration 0.6000g of khp so that the volume of naoh needed is 35.00ml

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Reagent grade potassium hydrogen phthalate (khp, mass 204.23g/mole) is a high molecular weight, stab...
Questions in other subjects:





Mathematics, 06.11.2020 04:10

English, 06.11.2020 04:10

Social Studies, 06.11.2020 04:10

Mathematics, 06.11.2020 04:10


Mathematics, 06.11.2020 04:10