
Chemistry, 14.07.2019 13:00 devenybates
At which temperature would a reaction with h = -92 kj/mol, s = -0.199 kj/(molk) be spontaneous?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
At which temperature would a reaction with h = -92 kj/mol, s = -0.199 kj/(molk) be spontaneous?...
Questions in other subjects:

Advanced Placement (AP), 30.01.2021 06:40


Mathematics, 30.01.2021 06:40

Mathematics, 30.01.2021 06:40

Mathematics, 30.01.2021 06:40



History, 30.01.2021 06:40

Biology, 30.01.2021 06:40

Mathematics, 30.01.2021 06:40

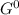 = Δ
= Δ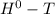 Δ
Δ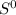
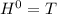 Δ
Δ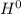 , giving a negative value for Δ
, giving a negative value for Δ 

