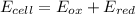Chemistry, 14.07.2019 14:30 sciencecreation87
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq) the reduction half-reaction has a standard potential of 1.18 v. the oxidation half-reaction has a standard potential of 0.34 v. what is the overall cell potential? (a) -1.52 v (b) -1.18 v (c) +1.18 v (d) +1.52 v

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq)...
Questions in other subjects:

Mathematics, 19.11.2019 03:31


Mathematics, 19.11.2019 03:31




Social Studies, 19.11.2019 03:31

Mathematics, 19.11.2019 03:31

Geography, 19.11.2019 03:31

History, 19.11.2019 03:31