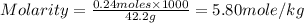Chemistry, 14.07.2019 19:30 MarishaTucker
Calculate the molality of a solution containing 14.3 g of nacl in 42.2 g of water. a. 2.45 ï´ 10â4 m b. 5.80 ï´ 10â4 m c. 2.45 ï´ 10â1 m d. 103 m e. 5.80 m

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


Chemistry, 22.06.2019 21:30, rondonalba
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2
You know the right answer?
Calculate the molality of a solution containing 14.3 g of nacl in 42.2 g of water. a. 2.45 ï´ 10â4 m...
Questions in other subjects:










Health, 17.02.2020 18:31

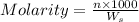
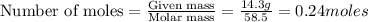
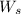 = weight of solvent in g= 42.2 g
= weight of solvent in g= 42.2 g