Chemistry, 14.07.2019 21:00 dbzrules02
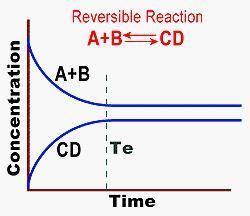
After reaching equilibrium, the rate of formation of products is less than the rate of formation of reactants. after reaching equilibrium, the rate of forming products and reactants is the same. equilibrium is obtained prior to te.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 09:30, Adolfosbaby
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
After reaching equilibrium, the rate of formation of products is less than the rate of formation of...
Questions in other subjects:

Biology, 03.12.2020 07:10



Mathematics, 03.12.2020 07:10

Physics, 03.12.2020 07:10



Health, 03.12.2020 07:10


Spanish, 03.12.2020 07:10