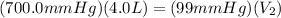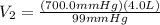Aballoon was partially filled with helium gas at room temperature. it occupied 4.0 liters of volume at 700.0 mmhg atmospheric pressure. when the balloon was released, it traveled upward until it burst at 99 mmhg atmospheric pressure. (neglect any force exerted to stretch the rubber balloon.) what was the volume of the balloon when it burst? l

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2



Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Aballoon was partially filled with helium gas at room temperature. it occupied 4.0 liters of volume...
Questions in other subjects:

Health, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32

Mathematics, 22.01.2020 06:32



English, 22.01.2020 06:32


English, 22.01.2020 06:32


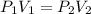
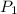 is the initial pressure and
is the initial pressure and 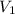 is initial volume.
is initial volume.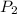 is final pressure and
is final pressure and 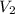 is final volume.
is final volume.