

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, cristinaledford3696
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3


Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
You know the right answer?
How many moles of ag can be produced if 350. g of cu are reacted with excess agno3 according to the...
Questions in other subjects:



English, 10.09.2019 05:30

History, 10.09.2019 05:30


Mathematics, 10.09.2019 05:30

Spanish, 10.09.2019 05:30


English, 10.09.2019 05:30

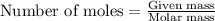
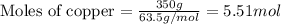
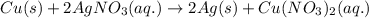
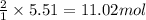 of silver.
of silver.

