
Chemistry, 15.07.2019 09:00 Jenniferwolf
How many moles of oxygen react with hydrogen to produce 22.8 mol of h2o? the balanced equation is as follows: 2h2 + o2 → 2h2o how many grams of co2 are produced if 35.48 mol of hcl are reacted according to this balanced chemical equation? caco3 + 2hcl → cacl2 + co2 + h2o

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1


You know the right answer?
How many moles of oxygen react with hydrogen to produce 22.8 mol of h2o? the balanced equation is a...
Questions in other subjects:



Mathematics, 21.09.2021 08:20



Mathematics, 21.09.2021 08:20

English, 21.09.2021 08:20


History, 21.09.2021 08:20

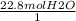 *
* 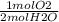 =
=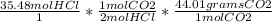 =
=

