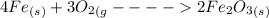Chemistry, 15.07.2019 10:30 munozgiselle
#11.) when iron rusts in air, iron(iii) oxide is produced. how many moles of oxygen react with 77.4 mol of iron in the rusting reaction? 4fe(s) + 3o2(g) → 2fe2o3(s) select one: a. 58 b. 100 c. 77 d. 120 #13.) for the reaction ch4 + 2o2 → co2 + 2h2o, how many moles of carbon dioxide are produced from the combustion of 161.0 g of methane? select one: a. 10.03 b. 20.06 c. 2584 d. 3.351

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
#11.) when iron rusts in air, iron(iii) oxide is produced. how many moles of oxygen react with 77.4...
Questions in other subjects:






Mathematics, 26.06.2019 22:20

Mathematics, 26.06.2019 22:20

History, 26.06.2019 22:20