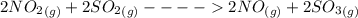Sulfuric acid was once produced through the reaction of sulfur trioxide with water. sulfur trioxide can form through the reaction of sulfur dioxide and oxygen gas. when nitrogen monoxide gas is added to the system, the reaction speeds up significantly because it proceeds through the following steps: 2no(g)+o2(> 2no2(g) 2no2(g)+> 2no(g)+2so3(g) identify the catalyst in this reaction, explain how you know it is the catalyst, and describe how it increases the rate of the reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 06:30, kaitlynk0
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Sulfuric acid was once produced through the reaction of sulfur trioxide with water. sulfur trioxide...
Questions in other subjects:




Social Studies, 06.03.2020 18:15


Mathematics, 06.03.2020 18:16



World Languages, 06.03.2020 18:16