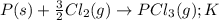Chemistry, 15.07.2019 13:00 pablogonzaleztellez
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction in terms of the equilibrium constants, ka and kb, for reactions a and b below: a.)p(s) + 5/2 cl2( g. pcl5( g. ka b.)pcl3( g. + cl2( g. pcl5( g. kb

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1


You know the right answer?
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction...
Questions in other subjects:

Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

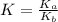
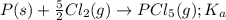
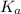 for the above equation is:
for the above equation is:![K_a=\frac{[PCl_5]}{[P][Cl_2]^{5/2}}](/tpl/images/0092/5571/bd7b7.png) ......(1)
......(1)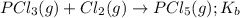
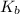 for the above equation is:
for the above equation is:![K_b=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0092/5571/bd7b0.png) ......(2)
......(2)![\frac{K_a}{K_b}=\left(\frac{\frac{[PCl_5]}{[P][Cl_2]^{5/2}}}{\frac{[PCl_5]}{[PCl_3][Cl_2]}}\right)\\\\\\\frac{K_a}{K_b}=\frac{[PCl_3]}{[P][Cl_2]^{3/2}}](/tpl/images/0092/5571/fcd9a.png)