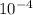Chemistry, 15.07.2019 19:00 lerasteidl
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) determine the direction of reaction if 1.00 m nocl is combined with 0.500 m no and 0.500 m cl2 in a sealed flask. a.)no reaction occurs. b.)reaction proceeds to the right toward the products. c.)reaction proceeds to the left toward the reactants. d.)reaction proceeds to the left toward the products.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2


Chemistry, 23.06.2019 05:40, Queenquestion9130
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) deter...
Questions in other subjects:





Mathematics, 27.02.2020 03:07

Mathematics, 27.02.2020 03:07

Mathematics, 27.02.2020 03:07

History, 27.02.2020 03:08

Mathematics, 27.02.2020 03:08

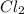
![Q = \frac{[ NO]^{2}. [Cl_{2} ]}{[ NOCl ]^{2}}](/tpl/images/1058/5527/aa67c.png)