
Chemistry, 16.07.2019 04:00 cmfield8810
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain why the shift occurs. b. what would have been observed if hcl (also a strong acid) had been added instead of the hno3?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain w...
Questions in other subjects:




Geography, 17.09.2019 09:50

Biology, 17.09.2019 09:50

History, 17.09.2019 09:50

Mathematics, 17.09.2019 09:50



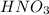 reacts with
reacts with 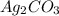 usually the equilibrium shifts to the right because
usually the equilibrium shifts to the right because 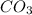 molecule from the reaction mixture, which then tends to shift the equilibrium towards right.
molecule from the reaction mixture, which then tends to shift the equilibrium towards right.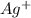 and
and 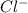 ions from the solution.
ions from the solution.

