
Chemistry, 16.07.2019 04:00 preciosakassidy
Asolution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0.250 g of the substance in 40.0 g of ccl4. the boiling point of the resultant solution was 0.357oc higher than that of the pure solvent. calculate the molar mass of the solute. (kb = 5.02oc/m)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, lifeofabe214
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1


Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
You know the right answer?
Asolution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0.250 g of the substan...
Questions in other subjects:



Mathematics, 09.06.2021 20:50


Mathematics, 09.06.2021 20:50

Physics, 09.06.2021 20:50



Mathematics, 09.06.2021 20:50

Mathematics, 09.06.2021 20:50

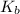 m
m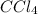 = 40,
= 40,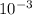 .
. 

