
Chemistry, 16.07.2019 09:30 manarsadi6
Achemical engineer determines the mass percent of iron in an ore sample by converting the fe to fe2+ in acid and then titrating the fe2+ with mno4−. a 1.3909−g sample was dissolved in acid and then titrated with 21.63 ml of 0.04462 m kmno4. the balanced equation is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

You know the right answer?
Achemical engineer determines the mass percent of iron in an ore sample by converting the fe to fe2+...
Questions in other subjects:


Mathematics, 27.04.2021 23:40

Mathematics, 27.04.2021 23:40

English, 27.04.2021 23:40


English, 27.04.2021 23:40

Mathematics, 27.04.2021 23:40

Mathematics, 27.04.2021 23:40

Business, 27.04.2021 23:40

Mathematics, 27.04.2021 23:40

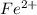 in acid and then titrating the
in acid and then titrating the 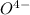 .
. 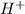 (aq) + 5
(aq) + 5 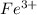 (aq) +
(aq) + 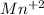 +(aq) + 4
+(aq) + 4 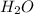 (l)
(l) 


