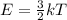Chemistry, 17.07.2019 04:30 thegreentnt5025
You are experimenting on the effect of temperature on the rate of reaction between hydrochloric acid (hcl) and potassium iodide (ki). when the reaction is completed at 273k there are approximately 250,000 collisions per mole of reactant. you run the experiment again at 350k. which of the following would you expect to be the number of collisions recorded at 350k a. 2000 b. 700,000 c. 0 d. 200,000

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
You are experimenting on the effect of temperature on the rate of reaction between hydrochloric acid...
Questions in other subjects:


Mathematics, 10.04.2020 03:54


English, 10.04.2020 03:54





Social Studies, 10.04.2020 03:55