

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
2hcl + mg mc031-1.jpg mgcl2 + h2 if 40.0 g of hcl react with an excess of magnesium metal, what is t...
Questions in other subjects:


Mathematics, 06.01.2021 17:40

Mathematics, 06.01.2021 17:40

Biology, 06.01.2021 17:40

Mathematics, 06.01.2021 17:40


Mathematics, 06.01.2021 17:40

Chemistry, 06.01.2021 17:40


Mathematics, 06.01.2021 17:40

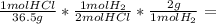 1.10 g H₂
1.10 g H₂

