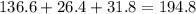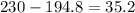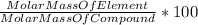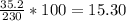Chemistry, 17.07.2019 07:30 sarahbennett11p4yxlb
A230 g sample of a compound contains 136.6 g of carbon, 26.4 g of hydrogen, and 31.8 g of nitrogen. the rest is oxygen. what is the mass percent of oxygen in the compound? 11.48%

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, acaciacoats
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
A230 g sample of a compound contains 136.6 g of carbon, 26.4 g of hydrogen, and 31.8 g of nitrogen....
Questions in other subjects:


Health, 15.07.2019 22:30




Mathematics, 15.07.2019 22:30


Biology, 15.07.2019 22:30

Mathematics, 15.07.2019 22:30