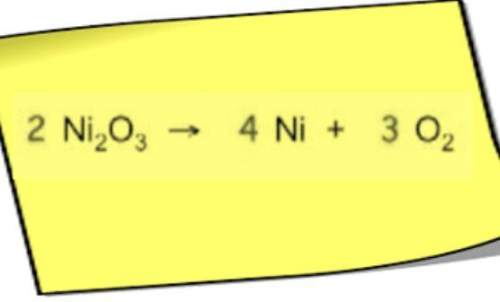
Chemistry, 17.07.2019 09:00 malachitorres813
Add the following quantities: 4.05 kg + 567.95 g + 100.1 g my solution: 4.05 + 567.95 + 100.1 = 672.1 there are several things wrong with this solution. tell me what they are.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, damienlopezram
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Add the following quantities: 4.05 kg + 567.95 g + 100.1 g my solution: 4.05 + 567.95 + 100.1 = 67...
Questions in other subjects:


Mathematics, 05.05.2020 17:20

Biology, 05.05.2020 17:20







English, 05.05.2020 17:20