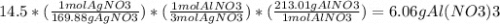Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

You know the right answer?
If 14.5 grams of silver nitrate (molar mass =169.88g/mol) reacts with aluminum chloride. how many gr...
Questions in other subjects:


English, 04.04.2020 18:16



Mathematics, 04.04.2020 18:16

History, 04.04.2020 18:16



Mathematics, 04.04.2020 18:16