

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Mercury (ii) sulfide, hgs h g s , contains 86.2\% 86.2 % mercury by mass. the mass of hgs h g s that...
Questions in other subjects:




Social Studies, 16.07.2019 14:00

Mathematics, 16.07.2019 14:00

Mathematics, 16.07.2019 14:00

History, 16.07.2019 14:00

Biology, 16.07.2019 14:00


Chemistry, 16.07.2019 14:00

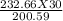 = 34.8 g
= 34.8 g


