
Chemistry, 18.07.2019 10:00 ssalusso1533
The first-order reaction, so2cl2 → so2 + cl2, has a rate constant equal to 2.20 × 10-5 s-1 at 593 k. what percentage of the initial amount of so2cl2 will remain after 2.00 hours?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
The first-order reaction, so2cl2 → so2 + cl2, has a rate constant equal to 2.20 × 10-5 s-1 at 593 k....
Questions in other subjects:



Geography, 12.10.2020 16:01

Advanced Placement (AP), 12.10.2020 16:01


Geography, 12.10.2020 16:01

Biology, 12.10.2020 16:01


History, 12.10.2020 16:01

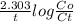
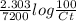
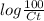 = 0.06877
= 0.06877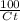 = 1.1716
= 1.1716


